The Medicare Drug Price Negotiation Program, enacted as part of the Inflation Reduction Act of 2022 (IRA), was designed to help lower Medicare spending on prescription drugs by requiring the federal government to negotiate the price of some high-cost drugs covered by Medicare. Along with lowering prices, the negotiation program could also improve coverage of drugs selected for negotiation for Medicare beneficiaries because the law requires all Medicare Part D plans to cover each of the selected drugs, including all dosages and forms, when negotiated prices take effect.
To measure the effect of the IRA’s coverage requirement for selected drugs, this analysis examines 2026 Medicare Part D formulary coverage of drugs selected for negotiation, including the first 10 drugs selected for price negotiation that now have Medicare-negotiated prices available as of January 1, 2026, and the second set of 15 drugs selected for price negotiation, whose negotiated prices will take effect in 2027. The analysis shows that for several dosages and forms of nine out of the first 10 selected drugs, coverage rates have improved since 2025, before the IRA’s coverage requirement took effect.
The IRA’s coverage requirement for selected drugs led to improved coverage of the Part D drugs with negotiated prices available in 2026
Consistent with the IRA’s coverage requirement, in 2026, all Part D enrollees have coverage of all 10 selected drugs with negotiated prices available this year, including all dosage forms and strengths. Moreover, access to several doses and forms of 9 of the first 10 drugs selected for negotiation has improved since 2025 (Figure 1). In particular, coverage rates of the insulin products Fiasp and NovoLog and two dosages of the cancer drug Imbruvica have expanded the most. Fiasp was covered for 24% of Part D enrollees in 2025, while NovoLog was covered for 32%, and two dosages of Imbruvica were covered for roughly half Part D enrollees in 2025.
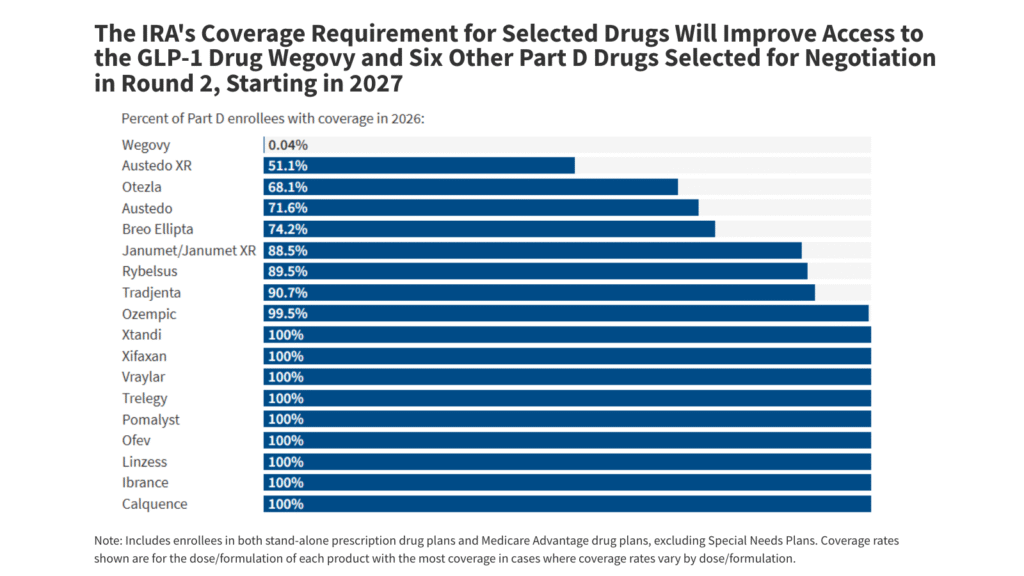
The IRA’s coverage requirement will improve coverage of several of the 15 selected drugs with negotiated prices available in 2027, including the GLP-1 drug Wegovy
The GLP-1 drug, Wegovy, one of the 15 selected drugs with negotiated prices available in 2027, is currently covered by a small number of Part D plans enrolling less than 1% of Part D enrollees in 2026 (Figure 2). Wegovy is approved for both obesity and cardiovascular disease risk reduction but Medicare Part D plans are currently allowed to cover Wegovy only for cardiovascular disease because Medicare is prohibited from covering drugs used for weight loss. The Trump administration is planning to launch a temporary, voluntary model to expand Medicare coverage of GLP-1s to treat obesity beginning in 2027, which would allow beneficiaries in participating Part D plans to use Wegovy for this purpose.
The IRA’s Part D coverage requirement for selected drugs will increase the share of Part D enrollees who have coverage of Wegovy for Medicare-covered uses beginning in 2027, along with 6 other selected drugs for 2027 that are not currently covered for all Part D enrollees, including Austedo and Austedo XR, a treatment for involuntary movement disorders (covered for 72% and 51% of enrollees, respectively); Otezla, a treatment for psoriasis and psoriatic arthritis (covered for 68% of enrollees); and Breo Ellipta, a treatment for asthma and COPD (covered for 74% of enrollees). At the same time, several of these drugs are already covered for all or nearly all Part D enrollees, including six drugs that belong to one of the six protected classes of drugs that are required to be covered by all plans: Xtandi, Pomalyst, Ofev, Ibrance, and Calquence are antineoplastics (drugs used to treat cancer) and Vraylar is an antipsychotic.
This work was supported in part by Arnold Ventures. KFF maintains full editorial control over all of its policy analysis, polling, and journalism activities.